Jenny Shelton takes us through the incredible potential of fungi and how this diverse, yet often overlooked, species may hold many of the answers to global challenges, from climate change to food insecurity.
Fungi play important roles in nature, food production, medicine, industry and bioremediation yet we have only discovered ~150,000 out of a potential 6.3 million fungal species. Just recently, a PhD student found undiscovered fungal species in seeds stored at the Millennium Seed Bank and estimates the whole collection might contain up to 1 million new species! You might be surprised to learn that fungi are more closely related to animals, and therefore humans, than they are to plants, and that the combined biomass of all fungi on Earth is 200 times greater than the entire human race.
The responses I often get when I tell someone I’m a mycologist is “Eurgh, I hate mushrooms!” or “Can you cure my athlete’s foot infection?”. It’s no surprise that this is their reaction given how little fungi feature in the U.K. school curriculum, or even in most biology and medical degrees, but it is such a shame given how magnificently diverse the Kingdom of Fungi is. (These responses also, unknowingly, capture the yin and yang of fungi: while this article is about the good that they can do it is important to acknowledge that fungal infections cause misery to millions of people around the world every year.)
Keeping in mind the challenges facing us – climate change, global food insecurity and infectious disease pandemics –I hope you will agree with me that the solutions to many of our problems could be fungal!
Fungi in nature
The fossil record shows us that fungi were one of the first land colonisers and that they formed symbiotic relationships with plants that lacked roots more than 475 million years ago. Together, these fungi and plants sequestered carbon dioxide (CO2) from the atmosphere and reduced it to levels that could sustain other life forms. This symbiosis continues today with around 80% of plants species forming interactions with mycorrhizal fungi; sequestering carbon from the air to exchange with fungi for nutrients such as nitrogen and phosphorus.
These underground fungal networks allow trees to warn each other of impending pest attacks and exchange nutrients, causing them to be nicknamed the “wood wide web”. In this way, they enhance the growth and survival of tree saplings – the mass-planting of which is being purported as a solution to climate change.
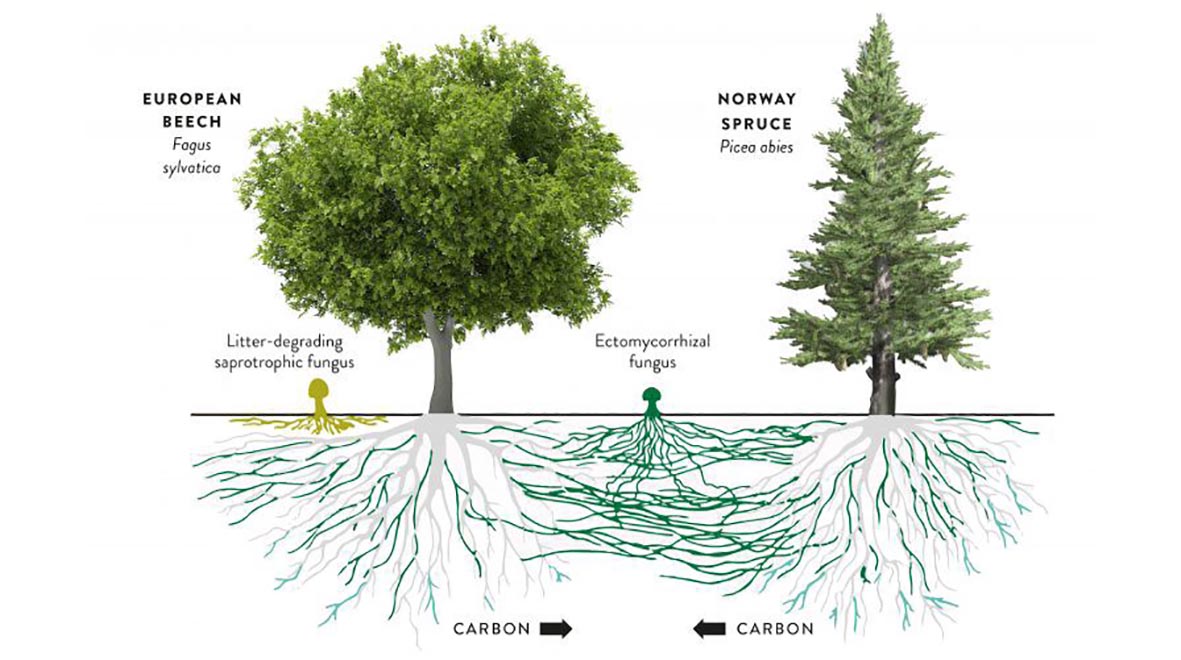
Mycorrhizal fungi are important for food security as they enhance growth of our main food crops rice, corn, soybean and wheat; by increasing their resistance to drought and disease, decreasing the need for fertilisers and alleviating stresses to the plant such as pollution and heavy metal contamination.
Soil fungi can also provide the solution to our pest problem, which is expected to worsen as global temperatures rise. At present, 40% of crops are lost to insects, pathogens and weeds and there is growing interest in using fungal properties for biocontrol – such as pesticides, fungicides and herbicides. With growing concerns over the impact of chemical interventions on water health and pollinator diversity, the fungal species that naturally occur in soil and rapidly control pest populations are a viable alternative.
Finally, saprotrophic fungi play an important role in the ecosystem by breaking down dead plant matter and recycling carbon and nutrients into the soil, such that life on earth without them would cease after a few decades when nutrients became unavailable. Through their decomposing activities fungi produce an organic material called humus, which increases the structure and water-holding capacity of soil and acts as the main carbon reservoir in the biosphere.
Fungi as food
When we talk about “mushrooms” we are referring to macrofungi, which are the fruiting bodies produced by fungi to disperse spores. Edible mushrooms are naturally high in vitamin D (one of few dietary sources of vitamin D suitable for vegans), low in calories, carbohydrates, calcium and sodium, and contain large numbers of vitamins and minerals. Commercial mushroom production has increased greater than 25-fold in the past 35 years, with button, oyster and Shiitake mushrooms accounting for 85% of worldwide consumption. Mushroom cultivation is low-cost and has low environmental impact so it is being explored as a solution to poverty and malnutrition in less economically developed countries.
Due to food shortages in the 1960s, a research program was established to look for sustainable sources of protein. They screened 3,000 soils from across the world and in a soil collected from a wheat field in Marlow, Buckinghamshire they found Fusarium venenatum, which produced a mycoprotein that is now known as Quorn™. Quorn™ is high in protein and fibre and low in fat, cholesterol, sodium and sugar, and many people eat it as a meat substitute to reduce their carbon footprint. One gram of mycoprotein is converted to 1,500 tonnes in each fermentation cycle, making it a highly sustainable and environmentally-friendly food source.
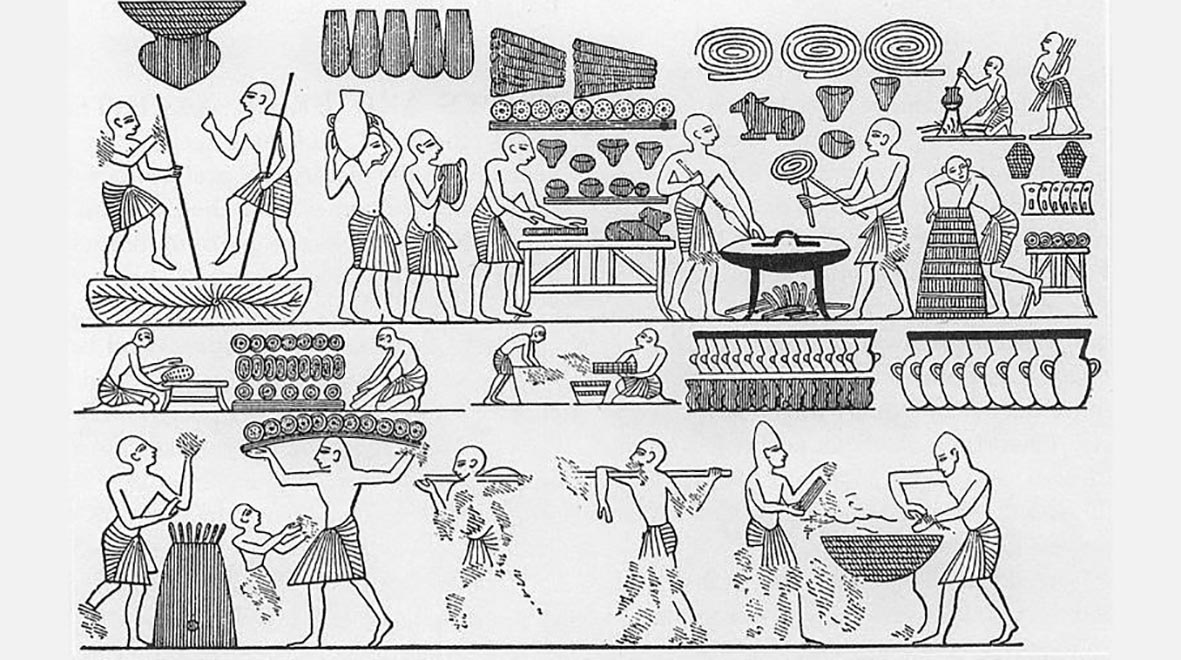
Perhaps the earliest known use of fungi in food production is baking and brewing using the yeast Saccharomyces cerevisae, which is first depicted in Egyptian hieroglyphs from 4,000 years ago. The importance of yeast comes from its ability to convert sugars into carbon dioxide and ethanol under both aerobic and anaerobic conditions, which is what causes bread dough to rise and makes wine and beer alcoholic.
If you like aged or fermented foods, it is likely a fungus is involved in its production. It’s probably no surprise that Pencillium roqueforti produces the blue veins in cheeses such as Roquefort, Stilton, and Gorgonzola, but you may not know that Penicillium camemberti produces the rind on soft cheeses like Camembert and Brie or that Penicillium salamii colonises the surface of dry-cured meats, enhancing their flavour and aroma and increasing their shelf-life by preventing spoilage by other microbes. Perhaps the clue is in the names! Many components of Asian cuisine are produced by fermentation using moulds: for example, wheat and soybean are fermented by Aspergillus species into soy sauce and rice is fermented by Aspergillus oryzae or Saccharomyces cerevisae into sake.
Fungi in medicine
Probably the best-known example of fungi in medicine is the discovery of penicillin by Sir Alexander Fleming in 1928 (at St Mary’s Hospital, so being a mycologist based at Imperial’s Paddington campus is brilliant!). The discovery happened by accident while Fleming was working on staphylococcal bacteria and noticed that mould contaminants on his agar plates inhibited bacterial growth around them. He nicknamed his finding ‘mould juice’, later naming it penicillin when he identified the mould as Penicillium, and found it to be active against all Gram-positive bacteria. Fleming was awarded the Nobel Prize for Medicine in 1945 and the discovery of penicillin triggered the beginning of the antibiotic era, which revolutionised the treatment of infectious diseases worldwide.

In the years since, many naturally-occurring fungal metabolites with antibacterial properties have been discovered, as well as antifungal, antiviral and antiprotozoal effects. It is estimated that by 2050, 10 million people will die each year from antimicrobial resistant infections if we do not develop new antimicrobial drugs. Fungi have likely developed an arsenal of antimicrobials over millions of years of fighting against their microbial competitors – we just need to tap into it!
Many fungi that have been used in traditional medicines since as far back as 3000 BC have anticancer effects; for example, lentinan produced by Shiitake mushrooms and polysaccharide peptide (PSP) produced by turkey tail mushrooms. Statins are produced by Penicillium and Aspergillus species and are best known for reducing deaths due to heart attacks and heart disease by reducing cholesterol levels, but can also inhibit acute myeloid leukaemia (AML), several cancers and melanomas.
Cyclosporine, produced by Tolypocladium inflatum, was the first metabolite produced by a microbe to be licensed for use as an immunosuppressant and is now used extensively in organ transplant patients to reduce graft rejection. Other immunosuppressants produced by fungi are used to treat autoimmune diseases such as Crohn’s disease, rheumatoid arthritis, lupus and psoriasis, as well as for organ transplants.
Finally, mushrooms that produce psilocybin (a.k.a. “magic mushrooms”) have been implicated in human evolution and have been found to have long-term antidepressant effects as well as treating tobacco and alcohol addiction and reducing symptoms of obsessive-compulsive disorder (OCD). The potential for using drugs such as psilocybin to treat intractable psychological disorders has led to burgeoning research in this area, such as the Centre for Psychedelic Research at Imperial College London.
Fungi in industry
You may have seen in the news that IKEA has replaced polystyrene with biodegradable mushroom-based packaging, Adidas has launched shoes made from mushroom leather and LEGO® uses itaconic acid to make its tree, leaf and bush components. These companies are committing to environmentally-friendly alternatives produced by or from fungi to reduce our reliance on fossil fuels and animal products. Materials made from fungal mycelium are also being explored for use in construction due to their high-tensile strength, acoustic absorbance and natural fire-retardance. As the production of mycelium is fast, requires low-energy input, can be grown on waste materials and does not create pollution, mycelial building materials could reduce the carbon footprint of building a house and replace building materials that are in short supply or are energy-intensive to produce.
During WWII, the uniforms and tents of Americans serving in the Pacific were disintegrating, which was found to be due to the fungus Trichoderma reesei producing cellulases that were breaking down cellulose fibres in the fabrics. Now cellulases, commercially produced by T. reesei and Aspergillus oryzae, account for approximately 14% of the global enzyme market and are used to give denim fabric a stone-washed look and eliminate hydrogen peroxide after it’s been used to bleach cotton. Lipases, commercially produced by Aspergillus niger, account for nearly 10% of the global enzyme market and are used in tanneries to hydrolyse fats, oils and greases present in hides and skins. Catalases and lipases produced by the thermophilic fungus Humicola insolens are added to biological washing powders to break down fat stains and to remove cotton threads from the surface of garments to make them feel smoother.
Fungi may even offer us a solution to our fossil fuel dilemma as they can convert cellulose substrates into mycodiesel and could use agricultural waste as a low cost and sustainable feedstock.
Fungi in bioremediation

Last, but by no means least, fungi can clean up the pollution we’ve caused around the world. Oyster mushrooms can grow in soils heavily contaminated with crude oil by breaking down the toxic, carcinogenic and mutagenic polycyclic hydrocarbons (PAH) within it. In one pilot study, the PAH content was reduced within several weeks to such levels that seeds were able to germinate and the Washington State of Department of Transport using the bioremediated soils for highway landscaping. As an added bonus, the spent mushroom waste (SMW) from the mushroom industry contains sufficient mycelia and nutrients for this purpose, thereby recycling a waste product for mycoremediation. Paul Stamets’ company Fungi Perfecti is currently testing MycoBooms™, which is a floating straw arm colonized with oyster mushrooms that could be used to contain and absorb oil spills in the sea.
In 2012, students of Yale University discovered a mushroom in the Amazon rainforest that can survive solely on plastic. In 2017, a fungus was discovered on a waste disposal site in Pakistan that was degrading polyurethane (PU), which accounts for 6-7% of total plastics produced worldwide. One idea that is currently being explored is incorporating a plastic-eating fungus into home recycling kits that would turn our plastic bags into food!
Fungi that can absorb radiocaesium have been found growing in the Shelter built around the damaged reactor of the Chernobyl Nuclear Power Plant and on land around Chernobyl and Fukushima power plants. There has been great interest in fungi as absorbents of environmental radionuclides due to the huge surface area of their hyphae and their ability to grow in the presence of radiation, heavy metals and low pH. Mushrooms that accumulate radionuclides prevent them from leaching into soil and can be removed and destroyed to prevent consumption by animals or humans, so they could play an important role in the bioremediation of radioactive sites.
NASA has even funded an astromycology project to discover fungi that are capable of surviving in space: to break down asteroid regolith and to establish healthy soils in extraterrestrial biospheres.
That was a whistle-stop tour through a lot of the incredible things that fungi are capable of and that we’ve been able to harness to help us in our everyday lives. I truly believe that the answers to most of our current and future problems lie beneath our feet in undiscovered soil fungi, in pristine forests and woodlands or our global banks of discovered fungi. I am also convinced that fungi hold the keys to curing human diseases, to breakdown our pollutants, to replace fossil fuels, to combat climate change and to provide our growing population with nutritious and sustainable food sources – we just need to unlock their potential. A global fungal discovery pipeline would not be too challenging to establish but the societal rewards could be transformational!
Jennifer Shelton (@jenmgshe) is a final-year PhD student with Professor Matthew Fisher in Imperial’s Department of Infectious Disease Epidemiology (DIDE) and Dr Andrew Singer at UK Centre for Ecology and Hydrology (UKCEH). She is part of the Science and Solutions for a Changing Planet (SSCP) DTP run by the Grantham Institute and funded by NERC.